Can the anomalous aqueous solubility of β-cyclodextrin be explained by its hydration free energy alone?†
Abstract
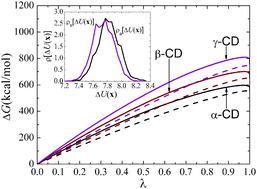
The well-documented anomalous solubility of β-cyclodextrin (β-CD), relative to α- and γ-CD, has been examined by Naidoo et al. (J. Phys. Chem. B, 2004, 108, 4236–4238.) from the perspective of water organization and internal motion of the macrocyclic rings. Whether modulation in the hydration patterns and in the rigidity of the molecular scaffold can be reconciled with the hydration free energy of β-CD to rationalize its notorious low solubility remains open to further investigation. In this contribution, multi-nanosecond molecular dynamics (MD) simulations have been carried out to investigate the hydration process of α-, β- and γ-CD. The distribution of

 Please wait while we load your content...
Please wait while we load your content...