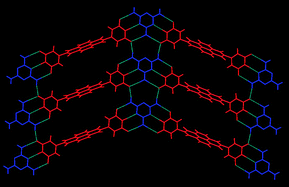
Three hydrogen-bonding tectons functionalised with pyrimidine-dione hydrogen-bonding moieties, either uracil or thymine groups, have been synthesised and their solid-state structures studied by single crystal X-ray diffraction. The tectons have different linkers between the pyrimidine-dione hydrogen-bonding groups. Whereas tectons 1 and 2 have either naphthalenetetracarboxylic diimide or p-xylyl spacers, respectively, linking two hydrogen-bonding appendages, tecton 3 comprises three thymine moieties linked via a 2,4,6-tris(methyl)mesitylene spacer. All three tectons have been structurally characterised and in the case of 2 and 3 exhibit the anticipated R22(8) double hydrogen-bonded arrangement between the imide moieties. In contrast, the single crystal structure of the DMSO solvate of 1 does not exhibit inter-tecton hydrogen-bonding but adopts N–H⋯O interactions with guest DMSO molecules. However, bimolecular hydrogen-bonded adducts have successfully been prepared exploiting the complementary triple hydrogen-bonding interactions between 1, or 2, and melamine to give two-dimensional hydrogen-bonded sheets.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...