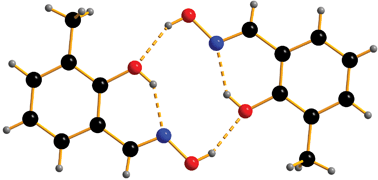
The effect of pressure on the crystal structures of 3-chloro-, 3-methoxy-, 3-methyl- and 3-tert-butylsalicylaldoximes has been investigated. The compounds all form the dimeric structure found in salicylaldoxime form I at ambient pressure, which is based on intermolecular hydrogen bonds between the oximic hydrogen and the phenolic oxygen atoms across an inversion centre. These intermolecular interactions, along with intramolecular phenolic hydrogen to oximic nitrogen atom hydrogen bonds form a pseudo-macrocycle with a R44(10) ring motif. These hydrogen bonding motifs pre-organize an arrangement of four potential donor atoms for a metal cation which, when the phenol groups are deprotonated, provides an N2O22− pocket well suited to the binding of planar transition metal ions. The radius of the cavity defined by the donor atoms in the dimers is dependent on the nature of the 3-substituent, varying from 1.949 Å in the 3-methoxy- to 2.037 Å in the 3-tBu-derivative. Anisotropic compression of the crystals on the application of pressure results in significant changes in the radii of the cavities in the dimers which decrease by ca. 10% at 6 GPa. During compression of the 3-tBu-derivative a single crystal to single crystal phase transition was observed between 0.2 and 1.0 GPa to a new polymorph, 3-tert-butylsalicylaldoxime-II. The phase transition produces an increase in symmetry as the space group changes from P-1 to I2/a, but the intermolecular interactions remain essentially unchanged. No phase transitions were observed in the compression of 3-Cl-, 3-Me- or 3-MeO-salicylaldoxime up to 6.2 GPa.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...