Immiscible electrolyte systems based on asymmetric hydrophobic room temperature ionic liquids
Abstract
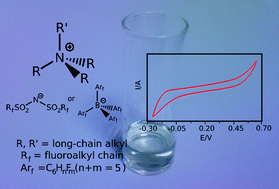
The effect on the melting point of the introduction of asymmetry in tetraalkylammonium halide salts has been investigated leading to the synthesis of new, hydrophobic (room temperature) ionic liquids suitable for liquid/liquid electrochemistry; one of these,

 Please wait while we load your content...
Please wait while we load your content...