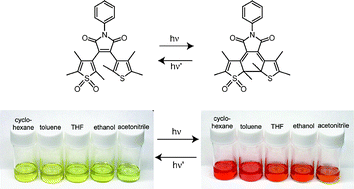
Photochromism of a symmetric diarylmaleimide derivative, having two thiophene rings (1), and a non-symmetric derivative having a S,S-dioxide thiophene ring and a thiophene ring (2) as the aryl moieties, was studied in various solvents. The photocyclization quantum yield of 1 gradually decreased with increasing the solvent polarity and the reaction was not observed in polar solvents, such as ethanol and acetonitrile; on the other hand, such a strong solvent dependence of the photocyclization reaction was not observed for 2; the different behavior is attributed to the weaker electron donating ability of the S,S-dioxide thiophene ring.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...