Formal total synthesis of triptolide†
Abstract
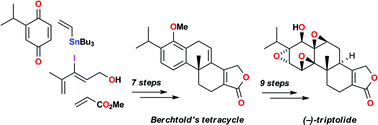
A new approach to the medicinally-important natural product triptolide is significantly shorter than previous syntheses, highly convergent and avoids the use of protecting groups; key features include two Diels–Alder reactions and a new deoxygenative aromatisation process.

 Please wait while we load your content...
Please wait while we load your content...