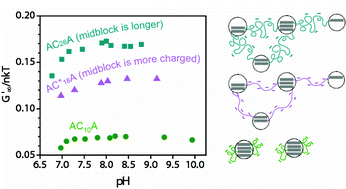
Artificial protein hydrogels made from a triblock protein (designated AC10A, where A is an acidic zipper domain and C10 comprises 10 repeats of the nonapeptide sequence 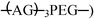 exhibit normalized plateau storage moduli (G′∞/nkT) less than 0.13 at all concentrations, pH values, and ionic strengths examined. These gels are surprisingly soft due to loop formation at the expense of bridges between physical junctions. Molecular-level evidence of loop formation is provided by strong fluorescence energy transfer (FRET) between distinct chromophores placed at the C- and N-termini of labelled chains diluted in an excess of unlabelled chains. The tendency to form loops originates from the compact size of the random coil midblock (mean RH(C10) ≈ 20 Å, determined from quasi-elastic light scattering of C10), and is facilitated by the ability of the leucine zipper domains to form antiparallel aggregates. Although the aggregation number of the leucine zipper domains is small (tetrameric, determined from multi-angle static light scattering of AC10 diblock), the average center-to-center distance between aggregates is roughly 1.5 times the average end-to-end distance of the C10 domain in a 7% w/v network. To avoid stretching the C10 domain, the chains tend to form loops. Changes in pH or ionic strength that expand the polyelectrolyte midblock favor bridging, leading to greater G′∞ as long as leucine zipper endblocks do not dissociate. Understanding of the network structure provided successful design strategies to increase the rigidity of these hydrogels. In contrast to intuitive design concepts for rubber and gel materials, it was shown that increasing either the length or the charge density of the midblock increases rigidity, because fewer chains are wasted in loop formation.
exhibit normalized plateau storage moduli (G′∞/nkT) less than 0.13 at all concentrations, pH values, and ionic strengths examined. These gels are surprisingly soft due to loop formation at the expense of bridges between physical junctions. Molecular-level evidence of loop formation is provided by strong fluorescence energy transfer (FRET) between distinct chromophores placed at the C- and N-termini of labelled chains diluted in an excess of unlabelled chains. The tendency to form loops originates from the compact size of the random coil midblock (mean RH(C10) ≈ 20 Å, determined from quasi-elastic light scattering of C10), and is facilitated by the ability of the leucine zipper domains to form antiparallel aggregates. Although the aggregation number of the leucine zipper domains is small (tetrameric, determined from multi-angle static light scattering of AC10 diblock), the average center-to-center distance between aggregates is roughly 1.5 times the average end-to-end distance of the C10 domain in a 7% w/v network. To avoid stretching the C10 domain, the chains tend to form loops. Changes in pH or ionic strength that expand the polyelectrolyte midblock favor bridging, leading to greater G′∞ as long as leucine zipper endblocks do not dissociate. Understanding of the network structure provided successful design strategies to increase the rigidity of these hydrogels. In contrast to intuitive design concepts for rubber and gel materials, it was shown that increasing either the length or the charge density of the midblock increases rigidity, because fewer chains are wasted in loop formation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
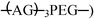 exhibit normalized plateau storage moduli (G′∞/nkT) less than 0.13 at all concentrations, pH values, and ionic strengths examined. These gels are surprisingly soft due to loop formation at the expense of bridges between physical junctions. Molecular-level evidence of loop formation is provided by strong fluorescence energy transfer (FRET) between distinct
exhibit normalized plateau storage moduli (G′∞/nkT) less than 0.13 at all concentrations, pH values, and ionic strengths examined. These gels are surprisingly soft due to loop formation at the expense of bridges between physical junctions. Molecular-level evidence of loop formation is provided by strong fluorescence energy transfer (FRET) between distinct 

 Please wait while we load your content...
Please wait while we load your content...