Intramolecular cyclization of β,β-difluorostyrenes bearing an iminomethyl or a diazenyl group at the ortho position: synthesis of 3-fluorinated isoquinoline and cinnoline derivatives
Abstract
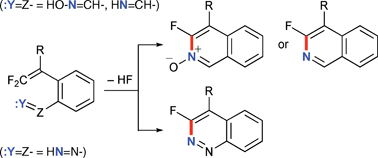
o-Formyl-substituted β,β-difluorostyrenes readily react with NH2OH·HCl or NH4OAc to afford ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– or HN
CH– or HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–). β,β-Difluorostyrenes bearing an o-diazenyl group (HN
CH–). β,β-Difluorostyrenes bearing an o-diazenyl group (HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), generated by
N–), generated by

 Please wait while we load your content...
Please wait while we load your content...