Evaluating β-amino acids as enantioselective organocatalysts of the Hajos–Parrish–Eder–Sauer–Wiechert reaction†
Abstract
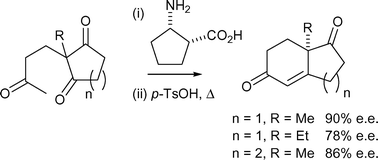
A systematic study of the effect of substitution within the β-amino acid framework indicates that both β2- and β3-amino acids catalyse the Hajos–Parrish–Eder–Sauer–Wiechert reaction with poor to reasonable levels of enantioselectivity. These results led to the evaluation of the conformationally constrained β-amino acid (1R,2S)-cispentacin, which catalyses the Hajos–Parrish–Eder–Sauer–Wiechert reaction with comparable or higher levels of enantioselectivity to L-proline.

 Please wait while we load your content...
Please wait while we load your content...