Asymmetric synthesis of 3,4-anti- and 3,4-syn-substituted aminopyrrolidinesvia lithium amide conjugate addition†
Abstract
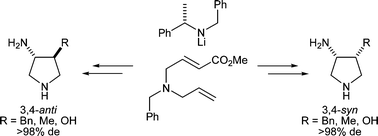
The diastereoselective conjugate addition of homochiral lithium amides to methyl 4-(N-allyl-N-benzylamino)but-2-enoate has been used as the key step in a simple and efficient protocol for the preparation of 3,4-substituted

 Please wait while we load your content...
Please wait while we load your content...