Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1,3-dipolar cycloaddition and their biological evaluation: implications for tumor targeting and tumor imaging purposes†‡
Abstract
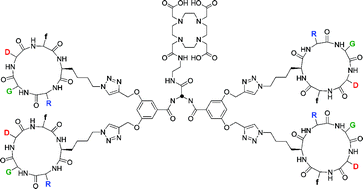
This report describes the design and synthesis of a series of αVβ3 integrin-directed monomeric, dimeric and tetrameric cyclo[Arg-Gly-Asp-D-Phe-Lys] dendrimers using “click chemistry”. It was found that the unprotected N-ε-azido derivative of cyclo[Arg-Gly-Asp-D-Phe-Lys] underwent a highly chemoselective conjugation to amino acid-based dendrimers bearing terminal alkynes using a microwave-assisted Cu(I)-catalyzed 1,3-dipolar cycloaddition. The αVβ3 binding characteristics of the dendrimers were determined in vitro and their in vivo αVβ3 targeting properties were assessed in nude mice with subcutaneously growing human SK-RC-52 tumors. The multivalent RGD-dendrimers were found to have enhanced affinity toward the αVβ3 integrin receptor as compared to the monomeric derivative as determined in an in vitro binding assay. In case of the DOTA-conjugated 111In-labeled RGD-dendrimers, it was found that the radiolabeled multimeric dendrimers showed specifically enhanced uptake in αVβ3 integrin expressing tumors in vivo. These studies showed that the tetrameric RGD-dendrimer had better tumor targeting properties than its dimeric and monomeric congeners.

 Please wait while we load your content...
Please wait while we load your content...