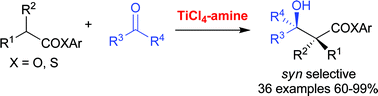
An efficient TiCl4–Et3N or Bu3N-promoted aldol-type addition of phenyl and thiophenyl esters or thioaryl esters with aldehydes and ketones was performed (total 46 examples). The present method is advantageous from atom-economical and cost-effective viewpoints; good to excellent yields, moderate to good syn-selectivity, substrate variations, reagent availability, and simple procedures. Utilizing the present reaction as the key step, an efficient short synthesis of three lactone [2(5H)-furanone] analogs of jasmine perfumes was performed. Among them, the lactone analog of cis-jasmone had a unique perfume property (tabac).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...