Synthesis of glycodendrimers containing both fucoside and galactoside residues and their binding properties to Pa-IL and PA-IIL lectins from Pseudomonas aeruginosa†‡
Abstract
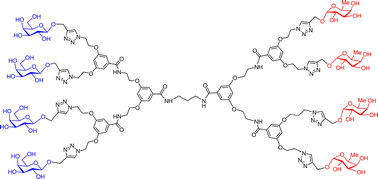
Homo- and hetero-bifunctional glycodendrimers ending with up to 16
- This article is part of the themed collection: Dendrimers

 Please wait while we load your content...
Please wait while we load your content...