Direct studies on 5-coordinate intermediates formed during substitution at tetrahedral Fe sites: role of bound nucleophile in labilisation of leaving group†
Abstract
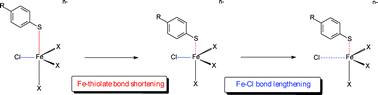
The substitution reactions of the tetrahedral Fe sites in [FeCl4]−, [Fe2S2Cl4]2−, [Fe4S4Cl4]2− and [{MoFe3S4Cl3}2(µ-SEt)3]3− with 4-RC6H4S− (R = MeO, Me, H, Cl or NO2) all involve rapid binding of the thiolate to a Fe site and formation of a kinetically and spectroscopically detectable intermediate. Kinetic studies allow calculation of the rate of Fe–Cl dissociation from the 5-coordinate site of the intermediate (k2R). The rate of Fe–Cl dissociation from the intermediate exhibits a marked dependence on the nature of the bound thiolate with log10(k2R) increasing in a linear manner with the calculated NBO charge on the sulfur atom of the coordinated thiolate. This behaviour indicates that Fe–Cl bond dissociation at the 5-coordinate intermediate involves a process in which Fe–thiolate bond shortening occurs prior to movement of the Fe–Cl bond.

 Please wait while we load your content...
Please wait while we load your content...