Supramolecular assemblies prepared from an iron(ii) tripodal imidazole complex. A molecular scaffolding for the self assembly of icosahedral complexes of K+, Rb+, Cs+ and NH4+ cations†‡
Abstract
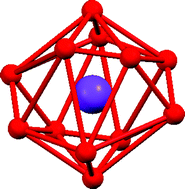
The iron(II) complex of the 1 : 3 Schiff base condensate of tris(2-aminoethyl)amine (tren) with 2-imidazolecarboxaldehyde, H3L1, [FeH3L1](ClO4)2, was reacted with MClO4 (M = K, Rb, Cs, and NH4). The products were double salts of the formula {[FeH3L1](ClO4)2}·MClO4. The complexes have been characterized by Elemental Analysis, X-ray crystallography, ESMS, IR and Mössbauer spectroscopy. The resultant complexes are all trigonal, crystallize in P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) and exhibit a hexameric structure in the extended lattice. The cations are located at the origin (0, 0, 0) and c/2 (0, 0, 0.5) on the c axis. The cations are twelve coordinate and exhibit a distorted icosahedral geometry formed by six bidentate perchlorate anions. The perchlorate anions are involved in extensive hydrogen bonding with the iron complexes, which are located at the vertices of the hexagon. Each perchlorate anion has five hydrogen bonds to three iron complexes. The iron complexes use the imidazole NH and imine N
and exhibit a hexameric structure in the extended lattice. The cations are located at the origin (0, 0, 0) and c/2 (0, 0, 0.5) on the c axis. The cations are twelve coordinate and exhibit a distorted icosahedral geometry formed by six bidentate perchlorate anions. The perchlorate anions are involved in extensive hydrogen bonding with the iron complexes, which are located at the vertices of the hexagon. Each perchlorate anion has five hydrogen bonds to three iron complexes. The iron complexes use the imidazole NH and imine N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH as donors to the perchlorate oxygen atoms. The imidazole NH is a trifurcated donor and the imine CH is a bifurcated donor. The formation of the double salt and the accompanying hydrogen bonding results in a shift in the spin equilibrium of the parent iron(II) complex from 63% high spin, HS, and 37% low spin, LS, at 295 K to pure LS in the double salts. Attempts to incorporate sodium or silver in the above salts failed due to their size and oxidizing strength, respectively. The analogous manganese complex, [MnH3L1](ClO4)2, failed to give these double salts under identical reaction conditions. The lack of reactivity of [MnH3L1](ClO4)2 does not appear to be due to its solubility or geometric considerations of the hydrogen bonding donor site, but instead to the electronic differences between the high spin [MnH3L1](ClO4)2 and the spin crossover [FeH3L1](ClO4)2.
CH as donors to the perchlorate oxygen atoms. The imidazole NH is a trifurcated donor and the imine CH is a bifurcated donor. The formation of the double salt and the accompanying hydrogen bonding results in a shift in the spin equilibrium of the parent iron(II) complex from 63% high spin, HS, and 37% low spin, LS, at 295 K to pure LS in the double salts. Attempts to incorporate sodium or silver in the above salts failed due to their size and oxidizing strength, respectively. The analogous manganese complex, [MnH3L1](ClO4)2, failed to give these double salts under identical reaction conditions. The lack of reactivity of [MnH3L1](ClO4)2 does not appear to be due to its solubility or geometric considerations of the hydrogen bonding donor site, but instead to the electronic differences between the high spin [MnH3L1](ClO4)2 and the spin crossover [FeH3L1](ClO4)2.

 Please wait while we load your content...
Please wait while we load your content...