Novel hydrido-ruthenium(ii) complexes with histidine derivatives and their application in the hydrogenation of ketones
Abstract
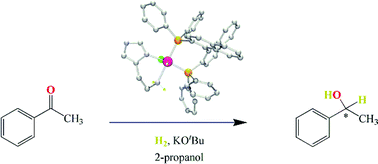
The complexes RuHCl((R)-binap)(L-NH2) with L-NH2 = (S)-histidine-Me-ester (1), histamine (3), (S)-histidinol (4) or 1-Me-(S)-histidine-Me-ester (5), and RuHCl((S)-binap)(L-NH2) with L-NH2 = (S)-histidine-Me-ester (2) have been prepared in 60–81% overall yields in a one-pot, three-step procedure from the precursor RuCl2(PPh3)3. Their octahedral structures with hydride trans to chloride were deduced from their NMR spectra and confirmed by the results of a single crystal X-ray diffraction study for complex 3. Under H2 and in the presence of KOtBu, complexes 1–5 in 2-propanol form moderately active catalyst precursors for the asymmetric hydrogenation of acetophenone to 1-phenylethanol. Complex 5 is more active and enantioselective than complexes 1–4, allowing complete conversion to 1-phenylethanol in 46% e.e. (R) in 72 h at 20 °C under 1 MPa of H2 with substrate : catalyst : base = 2000 : 1 : 30. Complex 5, when activated, also catalyzes the hydrogenation of trans-4-phenyl-3-buten-2-one to exclusively the allyl alcohol 4-phenyl-3-buten-2-ol under 2.7 MPa of H2 at 50 °C in 2-propanol. This selectivity for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O versus C
O versus C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenation is consistent with a mechanism involving the outer sphere transfer of hydride and proton to the polar bond. Further extensions to complexes with peptides with N-terminal histidine groups appear feasible on the basis of the current work.
C hydrogenation is consistent with a mechanism involving the outer sphere transfer of hydride and proton to the polar bond. Further extensions to complexes with peptides with N-terminal histidine groups appear feasible on the basis of the current work.

 Please wait while we load your content...
Please wait while we load your content...