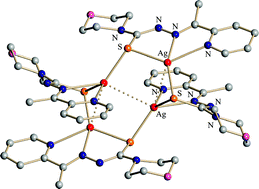
A novel neutral tetrameric silver(I) cluster [Ag(mtsc)]4 was obtained from reactions of a tridentate 4N-morpholyl 2-acetylpyridine thiosemicarbazone ligand (N′-[1-(2-pyridyl)ethylidene] morpholine-4-carbothiohydrazide, Hmtsc) and silver(I) sources containing Ag–O bonds (Ag2O, Ag(OAc), silver(I) 2-pyrrolidone-5-carboxylate ∞{[Ag(Hpyrrld)]2}, silver(I) 5-oxo-2-tetrahydrofurancarboxylate ∞{[Ag(othf)]2}, and silver(I) complexes with camphanic acid ∞{[Ag(ca)]} and ∞{[Ag(ca)(Hca)]}). The cluster was characterized by elemental analysis, TG/DTA, FTIR and single-crystal X-ray analysis in the solid state. The solution properties of the complexes were investigated using solution molecular weight measurement, ESI-MS and solution (1H, 13C and 31P) NMR spectroscopy. The obtained cluster is a novel example of a light-stable AgI cluster with a tridentate thiosemicarbazone ligand and the second report of a crystal structure of a thiosemicarbazone silver(I) complex. The reaction of the tetramer with a large excess of PPh3 gave dimeric complexes, namely, [Ag(µ(S)-mtsc)(PPh3)]2 and [(PPh3)2Ag(µ(S)-mtsc)2Ag]. The chloroform solution of the tetrameric complex showed modest and effective activities against selected bacteria (Bacillus subtilis, Staphylococcus aureus and Pseudomonas aeruginosa) and yeasts (Candida albicans and Saccharomyces cerevisiae), respectively, but it did not inhibit the growth of any selected microorganisms in a water-suspension system.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...