The first dinuclear copper(ii) and zinc(ii) complexes containing novel Bis-TACN: syntheses, structures, and DNA cleavage activities†
Abstract
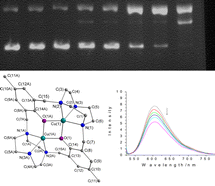
Two novel binuclear complexes [Cu2(L)]·(ClO4)2 (1) and [Zn2(L)]·(ClO4)2 (2) were synthesized and crystallographically characterized {L = 14,54-dimethyl-12,52-dihydroxy-1(1,3),5(1,3)-dibenzene-3(1,4),7(1,4)-di-1,4,7-triazacyclononane}. The cation [Cu2(L)]2+ structure of 1 is similar to that of [Zn2(L)]2+ of 2. The central ion is bridged by the di-phenoxo of L and lies in a close to perfect square pyramidal geometry. 1 and 2 crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The two complexes effectively promote the cleavage of plasmid DNA in the presence of activating agents at physiological pH and temperature. The pseudo-Michaelis–Menten kinetic parameters kcat = 1.61 h−1, Km = 1.35 × 10−5 M for complex 1 in the presence of mercaptoethanol; kcat = 2.48 h−1, Km = 5.5 × 10−5M for complex 2 in the presence of hydrogen peroxide were obtained. The mechanism of plasmid DNA cleavage was studied by adding standard radical scavengers. DNA cleavage reaction by the binuclear Zn(II)/H2O2 system is a hydrolytic mechanism.
. The two complexes effectively promote the cleavage of plasmid DNA in the presence of activating agents at physiological pH and temperature. The pseudo-Michaelis–Menten kinetic parameters kcat = 1.61 h−1, Km = 1.35 × 10−5 M for complex 1 in the presence of mercaptoethanol; kcat = 2.48 h−1, Km = 5.5 × 10−5M for complex 2 in the presence of hydrogen peroxide were obtained. The mechanism of plasmid DNA cleavage was studied by adding standard radical scavengers. DNA cleavage reaction by the binuclear Zn(II)/H2O2 system is a hydrolytic mechanism.

 Please wait while we load your content...
Please wait while we load your content...