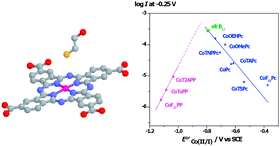
In this work we discuss different approaches for achieving electrodes modified with N4 macrocyclic complexes for the catalysis of the electrochemical oxidation of thiols. These approaches involve adsorption, electropolymerization and molecular anchoring using self assembled monolayers. We also discuss the parameters that determine the reactivity of these complexes. Catalytic activity is associated with the nature of the central metal, redox potentials and Hammett parameters of substituents on the ligand. Correlations between catalytic activity (log i at constant E) and the redox potential of catalysts for complexes of Cr, Mn, Fe, Co, Ni and Cu are linear with an increase of activity for more positive redox potentials. For a great variety complexes bearing the same metal center (Co) correlations between log i and Eo′ of the Co(II)/Co(I) couple have the shape of an unsymmetric volcano. This indicates that the potential of the Co(II)/Co(I) couple can be tuned using the appropiate ligand to achieve maximum catalytic activity. Maximum activity probably corresponds to a ΔG of adsorption of the thiol on the Co center equal to zero, and to a coverage of active sites by the thiol equal to 0.5.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...