Understanding the kinetics of spin-forbidden chemical reactions
Abstract
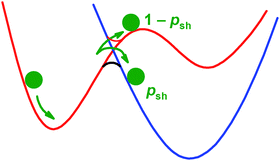
Many chemical reactions involve a change in spin-state and are formally forbidden. This article summarises a number of previously published applications showing that a form of Transition State Theory (TST) can account for the kinetics of these reactions. New calculations for the emblematic spin-forbidden reaction HC + N2 are also reported. The observed reactivity is determined by two factors. The first is the critical energy required for reaction to occur, which in spin-forbidden reactions is often defined by the relative energy of the Minimum Energy Crossing Point (MECP) between potential energy surfaces corresponding to the different spin states. The second factor is the probability of hopping from one surface to the other in the vicinity of the crossing region, which is largely defined by the spin–orbit coupling

 Please wait while we load your content...
Please wait while we load your content...