Contortions of encapsulated alkyl groups
Abstract
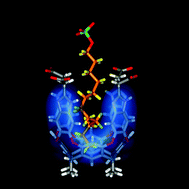
Rotors are recalled as early molecular devices that transmit information through changes in conformation. Specific cases involve bipyridyls and biphenyls in which the biaryl bond acts as a fulcrum to relay applied stresses from one site to another. New types of molecular stress encountered by encapsulated molecules are identified—including bending, straightening, squeezing, grinding and compression. For flexible molecules in reversibly formed capsules a fluid model of recognition is proposed that is neither lock-and-key nor induced fit. Instead, the guest assumes the shape that best fills the available space, even if contortions to higher energy conformations are required. For encapsulated alkanes, a delicate balance of attraction and repulsion exists when the size of a guest molecule approaches the space available to it. The complexes are analyzed by both NMR and computational methods and detailed maps of the host–guest interfaces in solution are provided. The reversible transition of an encapsulated alkane between a compressed, coiled conformation and a relaxed, extended one is described. The system is a spring-loaded molecular device under the control of acids and bases that offers an alternative to the rotors of current molecular machinery.

 Please wait while we load your content...
Please wait while we load your content...