Enantiospecific synthesis of the heparanase inhibitor (+)-trachyspic acid and stereoisomers from a common precursor†
Abstract
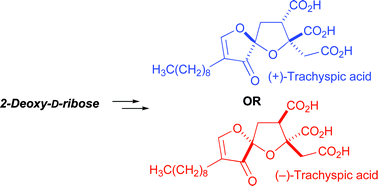
The total synthesis of natural (+)-trachyspic acid and its enantiomer is described starting from a common 2-deoxy-D-ribose derivative. The synthesis of the corresponding C3 epimers from the same starting material is also described. Each stereoisomer was assayed for

 Please wait while we load your content...
Please wait while we load your content...