Stereocontrol of intramolecular Diels–Alder reactions by an allylic diphenylcyclopropyl group†
Abstract
Intramolecular Diels–Alder reactions of ester-linked 1,3,8-nonatrienes carrying a diphenylcyclopropyl substituent attached to C1 proceed with high levels of stereoselectivity. The stereochemical outcomes of these reactions are explained by reference to B3LYP/6-31G(d) transition structures. Experimentally, the diphenylcyclopropane rings remain intact through these IMDA reactions, notwithstanding their predicted extremely high degree of asynchronicity (the B3LYP-computed lengths in the IMDA transition structures differ by as much as 1.1 Å), providing support to the notion that these reactions are concerted processes.
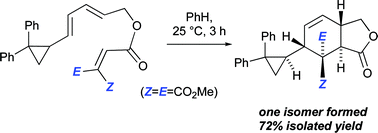

 Please wait while we load your content...
Please wait while we load your content...