Electrophilicity parameters for 2-benzylidene-indan-1,3-diones—a systematic extension of the benzhydrylium based electrophilicity scale†
Abstract
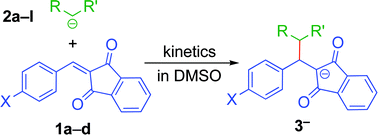
Kinetics of the reactions of four 2-benzylidene-indan-1,3-diones (1a–d) with carbanions (2a–l) have been studied photometrically in dimethyl sulfoxide solution at 20 °C, and the electrophilicity parameters E were determined by the linear free energy relationship log k2(20 °C) = s(N + E) (eqn (1)). The rate-determining step of these reactions is the nucleophilic attack of the carbon nucleophile at the double bond of the Michael acceptor. Comparisons with literature data show that the linear free energy relationship (eqn (1)) allows the semiquantitative prediction of the reactivities of 2-benzylidene-indan-1,3-diones towards various nucleophiles.

 Please wait while we load your content...
Please wait while we load your content...