Investigation of the synthetic and mechanistic aspects of the highly stereoselective transformation of α-thioamides to α-thio-β-chloroacrylamides†
Abstract
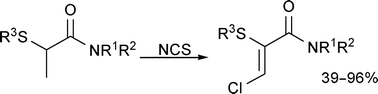
Treatment of a series of α-thioamides with N-chlorosuccinimide results in efficient transformation to the analogous α-thio-β-chloroacrylamides. The mechanistic pathway has been established through isolation and characterisation of intermediate compounds. The scope of the transformation has been explored—aryl and alkylthio substituents, primary, secondary and tertiary

 Please wait while we load your content...
Please wait while we load your content...