Abstract
Covering: up to 2006
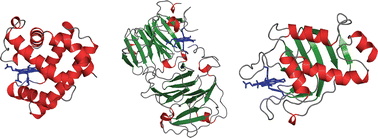
The heme prosthetic group is vital to many cellular processes and is therefore widespread throughout organisms of different phylogenetic origin. Heme is used in proteins involved in cellular respiration, acts as a chemical mediator in ligand binding and signalling proteins, and is the key co-factor in many enzymes. Strikingly, there are over 20 different folding topologies of b-type heme proteins that are able to incorporate the same, chemically identical heme ligand. Comparisons of structures show that heme–protein interactions are generally diverse, though a degree of conservation exists at contacts with the pyrrole rings, the propionate groups and the proximal ligand. These interaction “hot spots” presumably define major determinants for binding heme and provide guidelines for the future design of novel heme proteins.
- This article is part of the themed collection: Heme

 Please wait while we load your content...
Please wait while we load your content...