Carbo-mers: from skeleton to function
Abstract
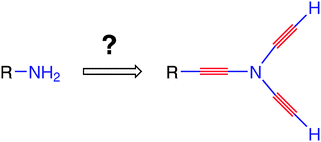
Since it has been proposed in 1995, the carbo-mer definition has been mainly applied to saturated and unsaturated rings of molecular skeletons, in view of studying their (homo)-aromatic character as compared to the electronic (de)localisation prevailing in the parent molecules. More generally, even acyclic or partial ethynylogues of common molecules exert fascination because the rigid expansion (preserving local symmetry) brought by the inserted acetylenic units gave them the status of “first-level nano-objects”. When the C2 insertion applies to the first shell of bonds at a given carbon or hetero-atom, the “exploded” unit can be considered a local carbo-mer of the corresponding function. The chemistry of molecules featuring a per-alkynylated atom is herein surveyed and perspectives are proposed. Some of them are “mysteriously” stable, while other remain “surprisingly” unknown in spite of their structural simplicity.

 Please wait while we load your content...
Please wait while we load your content...