Evaluating the cyclic π-electron delocalization energy through a double cut of conjugated rings
Abstract
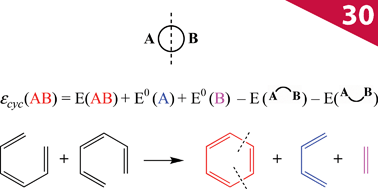
The cutting of two bonds of a ring A B allows us to define two closed-shell disconnected fragments A and B, and to evaluate the energy of the A
B allows us to define two closed-shell disconnected fragments A and B, and to evaluate the energy of the A B molecule from the eigenstates of A and B by second-order perturbation theory in the framework of tight-binding/Hückel theory. Diagrammatic arguments allow identification of the properly cyclic π-electron delocalization contribution to the energy, which we denote εcyc(AB), and which can be simply expressed in terms of the energies of the cyclic molecule, its fragments and of the two singly bridged
B molecule from the eigenstates of A and B by second-order perturbation theory in the framework of tight-binding/Hückel theory. Diagrammatic arguments allow identification of the properly cyclic π-electron delocalization contribution to the energy, which we denote εcyc(AB), and which can be simply expressed in terms of the energies of the cyclic molecule, its fragments and of the two singly bridged  and
and 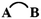 systems. The rules for the selection of suitable A and B fragments are given. The mathematical expression for εcyc(AB) can be translated into a chemical scheme that defines a new energetic aromaticity index, referred to as aromatic cyclic energy (ACE), which may be simply calculated from five Hückel energies. ACE of neutral and ionic [N]annulenes and their ring carbo-mers have been estimated, and shown to yield consistent evaluations of aromatic and antiaromatic cyclic energetic effects. A comparison is made with two other indices of aromaticity, Breslow resonance energies and ELFπ values.
systems. The rules for the selection of suitable A and B fragments are given. The mathematical expression for εcyc(AB) can be translated into a chemical scheme that defines a new energetic aromaticity index, referred to as aromatic cyclic energy (ACE), which may be simply calculated from five Hückel energies. ACE of neutral and ionic [N]annulenes and their ring carbo-mers have been estimated, and shown to yield consistent evaluations of aromatic and antiaromatic cyclic energetic effects. A comparison is made with two other indices of aromaticity, Breslow resonance energies and ELFπ values.

 Please wait while we load your content...
Please wait while we load your content...