Organic nanodots for multiphotonics: synthesis and photophysical studies†
Abstract
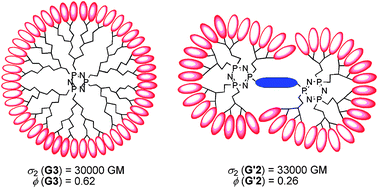
Organic nanodots based on the gathering of an exponentially increasing number of two-photon fluorophores on a dendritic platform of controlled size and symmetry represent a promising non-toxic alternative to
- This article is part of the themed collection: Dendrimers

 Please wait while we load your content...
Please wait while we load your content...