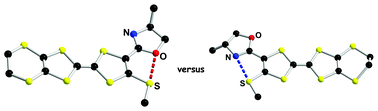
Racemic and enantiopure ethylenedithio-tetrathiafulvalene-thiomethyl-oxazoline (EDT-TTF-(SMe)-OX) derivatives have been synthesized. Single-crystal X-ray diffraction analyses reveal the establishment of O⋯S nonbonded interactions, unprecedented in the TTF series, characterized by short S⋯O distances and linear O⋯SMe motifs. Theoretical calculations at the DFT/B3LYP level on a model molecule demonstrate that both possible planar s-trans (O⋯S interaction) and s-cis (N⋯S interaction) conformations are energy minima, separated by an extremely weak energy gap. The energy barrier corresponding to the equilibrium between the two forms has been also estimated from DFT calculations, as well as their relative stability in the radical cation state of the TTF. According to the latter, there is a slight tendency towards the enhancement of the N⋯S interactions. Following the predictions of the theoretical calculations, the coexistence of both O⋯S and N⋯S nonbonded interactions is observed in two mixed valence radical cation salts of the racemic TTF with the dianionic cluster Mo6Cl14, prepared upon electrocrystallization. Interestingly, the ratio TTF : dianion is finely tuned by the choice of the electrocrystallization solvent. Physical measurements such as electrical conductivity and thermoelectric power on single crystals combined with magnetic susceptibility data in one case and extended Hückel tight-binding calculations demonstrate and rationalize the semiconducting behavior of both mixed valence salts. This study demonstrates that intramolecular N⋯S and O⋯S interactions can efficiently modulate and direct in the TTF series the occurrence of original solid-state structures provided with physical properties.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...