A single-molecular pathway from heterometallic MM′ (M = Baii, Mnii; M′ = Criii) oxalato complexes to intermetallic composite oxides
Abstract
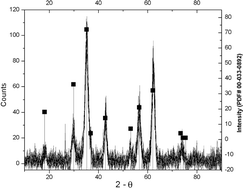
Thermolysis of {(n-C4H9)4N[MnIICrIII(C2O4)3]}n, 1 at 500 °C for 10 h gives spinel Mn1.5Cr1.5O4 which was characterized by powder XRD, SEM, FTIR and elemental analysis. The thermal conversion occurs by an internal redox process at ca. 400 °C in one step (TGA). It offers an alternative molecular source for an intermetallic oxide. Thermolysis of {[BaII6(H2O)17][CrIII(C2O4)3]4}·7H2O, 2 under similar conditions gives a mixture of BaIICrVIO4 and BaIICO3. These results suggested that the oxalate ligand in a heterometallic complex may be a convenient source for oxides in intermetallic composites and that, under suitable conditions, both metals in the heterometallic complexes can be transferred to the intermetallic oxides. It suggested that composite metal oxides can be generated from hetero- and inter-metallic oxalato complexes at relatively low temperatures, which could serve as a convenient route for the preparation of technologically important composites.

 Please wait while we load your content...
Please wait while we load your content...