Synthesis of ionic liquids in micro-reactors—a process intensification study
Abstract
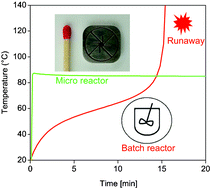
To date the manufacturing of ionic liquids on a large scale is limited by ineffective batch procedures employed for the alkylation step. Here we present a way to intensify the synthesis of 1-butyl-3-methylimidazolium bromide ([BMIM]Br) by using a continuously operating micro-reactor system. It consists of a microstructured mixer of 450 µm channel width and reaction tubes with inner diameter ranging from 2 to 6 mm allowing a production rate of 9.3 kg [BMIM]Br per day. In this reactor system the strongly exothermic alkylation can be thermally controlled even at elevated temperatures leading to high reaction rates in a solvent-free modus. Inspite of temperatures up to 85 °C the product purity achieved was above 99%. The degree of process intensification achieved results in a more than twentyfold increase of the space–time–yield compared to a conventional batch process. The measured conversion data could be modelled successfully using a second order reaction kinetic. With the generated kinetic parameters the time course of temperature and conversion was also simulated for batch synthesis. Based on these data the performance of the continuous micro-reactor and the conventional batch process was compared. The simulation shows the potential of process intensification as an improvement of space–time–yield in the range of two orders of magnitude.

 Please wait while we load your content...
Please wait while we load your content...