Molecular tectonics: on the formation of 1-D silver coordination networks by thiacalixarenes bearing nitrile groups†‡
Abstract
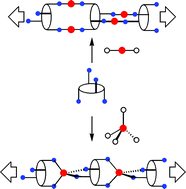
Two new p-tert-butylthiacalix[4]arene derivatives 2 and 3 decorated at the lower rim with four nitrile groups have been prepared and structurally characterised in the crystalline phase. The two

 Please wait while we load your content...
Please wait while we load your content...