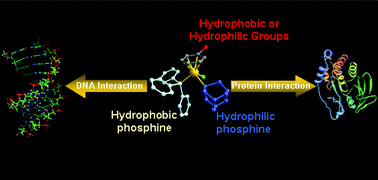
The antitumour activity of the organometallic ruthenium(II)–arene mixed phosphine complexes, [Ru(η6-p-cymene)Cl(PTA)(PPh3)]BF41b and [Ru(η6-C6H5CH2CH2OH)Cl(PTA)(PPh3)]BF42b (PTA = 1,3,5-triaza-7-phosphaadamantane), have been evaluated in vitro and compared to their RAPTA analogues, [Ru(η6-p-cymene)Cl2(PTA)] 1a and [Ru(η6-C6H5CH2CH2OH)Cl2(PTA)] 2a. The results show that the addition of the PPh3 ligand to 2a increases the cytotoxicity towards the TS/A adenocarcinoma cancer cells, which correlates with increased uptake, but also increases cytotoxicity to non-tumourigenic HBL-100 cells, thus decreasing selectivity. The decrease in selectivity has been correlated to increased DNA interactions relative to proteins, demonstrated by reactivity of the compounds with a 14-mer oligonucleotide and the model proteins ubiquitin and cytochrome-c.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...