Tuning Lewis acidity using the reactivity of “frustrated Lewis pairs”: facile formation of phosphine-boranes and cationic phosphonium-boranes†
Abstract
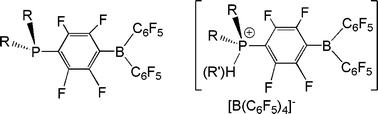
The concept of “frustrated Lewis pairs” involves donor and acceptor sites in which steric congestion precludes Lewis acid–base adduct formation. In the case of sterically demanding

 Please wait while we load your content...
Please wait while we load your content...