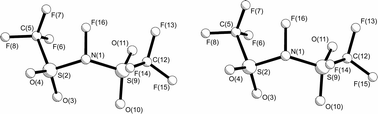
The structure of N-fluorobis(trifluoromethylsulfonyl)imide, prepared by a relatively safe and easy method, has been determined by gas-phase electron diffraction (GED), employing the SARACEN method, with flexible restraints based on the MP2/6-311G* structure, and by X-ray crystallography at 150 K. The strongly electron-withdrawing CF3 and SO2CF3 groups make the C–S and N–S distances long, averaging 187.7(3) and 171.7(3) pm, respectively, in the gas phase. The gas consists of two conformers, one (75%) with a CF3 group on each side of the SNS plane, one anti-periplanar and one syn-periplanar to the further N–S bond (ap, sp), and the other with both CF3 groups on the same side, i.e. denoted ap, ap. These conformers have very different SNS angles, 126.9(9)° and 117.1(17)° respectively. In the crystal all molecules have the ap, sp conformation, with parameters similar to those found for this conformer in the gas phase.

 Please wait while we load your content...
Please wait while we load your content...