Adsorption properties of HKUST-1 toward hydrogen and other small molecules monitored by IR
Abstract
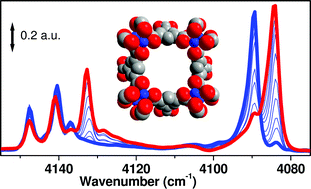
Among microporous systems metal organic frameworks are considered promising materials for molecular adsorption. In this contribution infrared spectroscopy is successfully applied to highlight the positive role played by coordinatively unsaturated Cu2+ ions in HKUST-1, acting as specific interaction sites. A properly activated material, obtained after solvent removal, is characterized by a high fraction of coordinatively unsaturated Cu2+ ions acting as preferential adsorption sites that show specific activities towards some of the most common gaseous species (NO, CO2, CO, N2 and H2). From a temperature dependent IR study, it has been estimated that the H2 adsorption energy is as high as 10 kJ mol−1. A very complex spectral evolution has been observed upon lowering the temperature. A further peculiarity of this material is the fact that it promotes ortho–para conversion of the adsorbed H2 species.

 Please wait while we load your content...
Please wait while we load your content...