Kinetic measurements of hydrocarbon conversion reactions on model metal surfaces†
Abstract
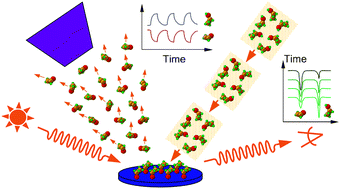
Examples from recent studies in our laboratory are presented to illustrate the main tools available to surface scientists for the determination of the kinetics of surface reactions. Emphasis is given here to

 Please wait while we load your content...
Please wait while we load your content...