SuperQuat 5,5-dimethyl-4-iso-propyloxazolidin-2-one as a mimic of Evans 4-tert-butyloxazolidin-2-one†
Abstract
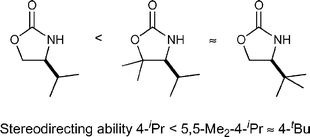
The incorporation of a gem-dimethyl group at the 5-position of a chiral oxazolidinone biases the conformation of the adjacent C(4)-stereodirecting group such that the gem-dimethyl-4-iso-propyl combination mimics a C(4)-tert-butyl group, providing higher levels of stereocontrol than a simple 4-iso-propyloxazolidinone. The generality of this principle is demonstrated with applications in stereoselective enolate alkylations, kinetic resolutions, Diels–Alder cycloadditions and Pd-catalysed asymmetric acetalisation reactions.

 Please wait while we load your content...
Please wait while we load your content...