Oxazinanones as chiral auxiliaries: synthesis and evaluation in enolate alkylations and aldol reactions
Abstract
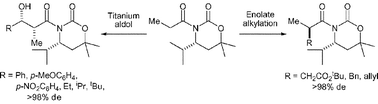
Homochiral β-amino esters (prepared on multigram scale by lithium amide conjugate addition) are readily transformed into oxazinanones. N-Acyl derivatives of oxazinanones undergo stereoselective enolate alkylation reactions, with higher stereoselectivities observed for the enolate alkylation of (R)-N-propanoyl-4-iso-propyl-6,6-dimethyl-oxazinan-2-one than the corresponding Evans oxazolidin-2-one. A C(4)-iso-propyl stereodirecting group within the oxazinanone conveys higher stereoselectivity than the analogous C(4)-phenyl substituent. gem-Dimethyl substitution at C(6) within the oxazinanone framework facilitates exclusive exocyclic cleavage upon hydrolysis to furnish α-substituted carboxylic acid derivatives and the parent oxazinanone in good yield. Asymmetric aldol reactions of a range of aromatic and aliphatic aldehydes with the chlorotitanium enolate of (R)-N-propanoyl-4-iso-propyl-6,6-dimethyl-oxazinan-2-one proceed with excellent diastereoselectivity. Hydrolysis of the aldol products affords homochiral α-methyl-β-hydroxy-carboxylic acids.

 Please wait while we load your content...
Please wait while we load your content...