Synthesis of iso-epoxy-amphidinolide N and des-epoxy-caribenolide I structures. Initial forays
Abstract
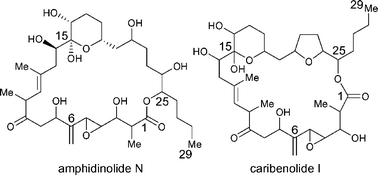
Two strategies for the projected total synthesis of the phenomenally potent antitumour macrolides amphidinolide N (1) and caribenolide I (2) are described. The title compounds are introduced as challenging and unique targets for chemical synthesis, and their retrosynthetic analysis is presented. The synthesis of the four defined key building blocks (10, 39, 67 and 72), required for the construction of amphidinolide N (1), in their enantiomerically pure forms, is described, followed by the coupling of 10, 39 and 72 through hydrazone alkylation processes to generate the complete C6–C29 carbon framework of the target compound (1). Fusion of the remaining C1–C5 sector (72) onto the molecule by metathesis-based methods was unsuccessful, resulting in the adoption of a second-generation strategy which called for the employment of one of the array of palladium-catalysed cross-coupling reactions to generate the C5–C6 carbon–carbon bond. Vinyl bromide 125, representing the C6–C29 skeleton of caribenolide I (2), was prepared through the sequential alkylation of hydrazone 10 with bromide 116 and iodide 55, but failed to engage in the appropriate cross-coupling reaction with a variety of C1–C4 partners. Despite these setbacks, the information gleaned from these endeavours was to prove invaluable in laying the foundation for the eventual successful approach to the macrocyclic structures of amphidinolide N (1) and caribenolide I (2).

 Please wait while we load your content...
Please wait while we load your content...