Nitrobenzoxadiazoles and related heterocycles: a relationship between aromaticity, superelectrophilicity and pericyclic reactivity†
Abstract
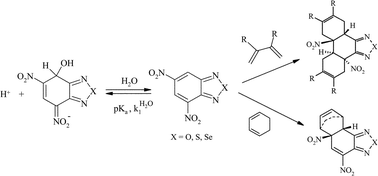
A study of the dual electrophilic and pericyclic reactivity of 4,6-dinitrobenzofurazan (DNBZ, 2), 4,6-dinitro-2,1,3-benzothiadiazole (DNBS, 3), 4,6-dinitro-2,1,3-benzoselenadiazole (DNBSe, 4) is reported. Kinetic and thermodynamic measurements of the ease of covalent hydration of 2–4 to give the corresponding hydroxy σ-adducts C-2–C-4 have been carried out over a large pH range in aqueous solution. Analysis of the data has allowed a determination of the rate constants 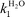 pertaining to the susceptibility of 2–4 to water attack as well as the pKa values for the σ-complexation processes. With pKa values ranging from 3.92 for DNBZ to 6.34 for DNBSe to 7.86 for DNBS, the electrophilic character of the three heteroaromatics is much closer to that of the superelectrophilic reference, i.e. 4,6-dinitrobenzofuroxan (DNBF, 1; pKa = 3.75), than that of the standard Meisenheimer electrophile 1,3,5-trinitrobenzene (TNB, pKa = 13.43). Most importantly, water is found to be an efficient nucleophile which contributes strongly to the formation of the adducts C-2 and C-4. This confirms a previous observation that a pKa value of ca. 8 is a primary requirement for having H2O competing effectively as a nucleophile with OH− in the formation of hydroxy σ-adducts. On the other hand, 2–4 are found to exhibit dienophilic and/or heterodienic behaviour on treatment with isoprene, 2,3-dimethylbutadiene, cyclopentadiene or cyclohexadiene, affording Diels–Alder mono- or di-adducts which have all been structurally characterized. A major finding is that the order of Diels–Alder reactivity follows clearly the order of electrophilicity, pointing to a direct relationship between superelectrophilic and pericyclic reactivity. This relationship is discussed.
pertaining to the susceptibility of 2–4 to water attack as well as the pKa values for the σ-complexation processes. With pKa values ranging from 3.92 for DNBZ to 6.34 for DNBSe to 7.86 for DNBS, the electrophilic character of the three heteroaromatics is much closer to that of the superelectrophilic reference, i.e. 4,6-dinitrobenzofuroxan (DNBF, 1; pKa = 3.75), than that of the standard Meisenheimer electrophile 1,3,5-trinitrobenzene (TNB, pKa = 13.43). Most importantly, water is found to be an efficient nucleophile which contributes strongly to the formation of the adducts C-2 and C-4. This confirms a previous observation that a pKa value of ca. 8 is a primary requirement for having H2O competing effectively as a nucleophile with OH− in the formation of hydroxy σ-adducts. On the other hand, 2–4 are found to exhibit dienophilic and/or heterodienic behaviour on treatment with isoprene, 2,3-dimethylbutadiene, cyclopentadiene or cyclohexadiene, affording Diels–Alder mono- or di-adducts which have all been structurally characterized. A major finding is that the order of Diels–Alder reactivity follows clearly the order of electrophilicity, pointing to a direct relationship between superelectrophilic and pericyclic reactivity. This relationship is discussed.

 Please wait while we load your content...
Please wait while we load your content...