Nucleoside synthesis from 3-alkylated sugars: role of 3β-oxy substituents in directing nucleoside formation
Abstract
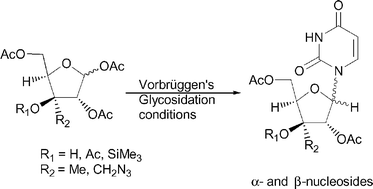
Using Vorbrüggen's protocol, reaction of persilylated uracil with xylofuranose derivatives having 3β-oxy-3α-alkyl substitution produced both α- and β-nucleosides. Only the β-nucleosides were formed from substrates having the reverse stereochemistry at C-3 or lacking the 3-alkyl substituent. Participation of the 3β-oxy substituent in stabilizing the incipient C-1 carbonium ion (or oxonium ion) intermediate has been suggested from analysis of energy-minimized conformations.

 Please wait while we load your content...
Please wait while we load your content...