Chemoenzymatic synthesis of GM3 and GM2 gangliosides containing a truncated ceramide functionalized for glycoconjugate synthesis and solid phase applications†
Abstract
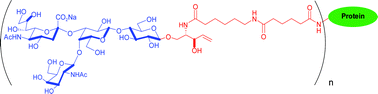
Analogues of GM3 and GM2 gangliosides were chemoenzymatically synthesized on a multifunctional ceramide-type tether designed to facilitate diverse strategies for glycoconjugate synthesis. The truncated ceramide aglycon maintains the stereogenic centres of natural ceramide while avoiding extensive hydrophobicity that can hamper synthesis and purification of the glycolipids. Tetanus toxoid and BSA glycoconjugates of these two gangliosides were prepared for immunization of mice, and for solid phase assays to screen for ganglioside-specific antibodies. Inhibition experiments showed that antibodies generated by tetanus toxoid conjugates of GM3 and GM2 exhibited specificity for the carbohydrate epitope and the stereogenic centres of the ceramide.

 Please wait while we load your content...
Please wait while we load your content...