Hydrogen adsorption on magnesium-exchanged zeolites
Abstract
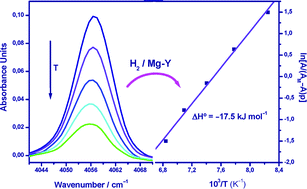
By means of variable-temperature FTIR spectroscopy, the standard adsorption enthalpy of hydrogen on the zeolite (Mg,Na)-Y was found to be ΔH° = −17.5 kJ mol−1, which suggests that magnesium-containing porous materials are good candidates in the search for suitable adsorbents for reversible hydrogen storage.

 Please wait while we load your content...
Please wait while we load your content...