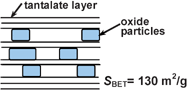
The Ruddlesden–Popper-type layered perovskite tantalates, H2ATa2O7 (A = Sr or La2/3), were pillared with n-alkylamines and oxide nanoparticles for the first time. H2SrTa2O7 can accommodate n-alkylamines (carbon numbers = 4, 8, 12, 16) to form intercalation compounds. A linear relationship is observed between the interlayer distance and the number of carbon atoms in n-alkyl chains, indicating the formation of a bilayer of the n-alkylamines. Porous metal oxides were synthesized by pillaring the n-octylamine intercalated H2ATa2O7 perovskites with Fe2O3 or Fe–Si mixed oxide (FeSi). These materials were well characterized by XRD, TEM, N2 adsorption, and Fe K-edge XAFS (XANES and EXAFS). FeSi pillared H2SrTa2O7 and H2La2/3Ta2O7 have a high surface area (52 and 130 m2 g−1) and microporosity. Fe2O3 pillared H2SrTa2O7 has a macroporous structure with a relatively low surface area (14 m2 g−1). XAFS analysis reveals that the Fe species in FeSi pillared perovskites are tetrahedral Fe3+ species incorporated in a silica matrix or highly dispersed on silica particles, while those in the Fe2O3 pillared one are α-Fe2O3 nanoparticles. The acidic property is tested by temperature programmed desorption (TPD) of ammonia, and the result shows that pillaring increases the number of acid sites in perovskites.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...