Synthetic and structural studies on C-ethynyl- and C-bromo-carboranes†
Abstract
A high-yield preparation of the C-monoethynyl para-carborane, 1-Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-1,12-C2B10H11, from C-monocopper para-carborane and
C-1,12-C2B10H11, from C-monocopper para-carborane and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 is reported. The low-yield preparation of 1,12-(Me3SiC
CSiMe3 is reported. The low-yield preparation of 1,12-(Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2-1,12-C2B10H10 from the C,C′-dicopper para-carborane derivative with
C)2-1,12-C2B10H10 from the C,C′-dicopper para-carborane derivative with ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3, has been re-investigated and other products were identified including the C-monoethynyl-carborane 1-Me3SiC
CSiMe3, has been re-investigated and other products were identified including the C-monoethynyl-carborane 1-Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-1,12-C2B10H11 and two-cage assemblies generated from cage–cage couplings. The contrast in the yields of the monoethynyl and diethynyl products is due to the highly unfavourable coupling process between 1-RC
C-1,12-C2B10H11 and two-cage assemblies generated from cage–cage couplings. The contrast in the yields of the monoethynyl and diethynyl products is due to the highly unfavourable coupling process between 1-RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-12-Cu-1,12-C2B10H10 and the bromoalkyne. The ethynyl group at the cage carbon C(1) strongly influences the chemical reactivity of the cage carbon at C(12)—the first example of the ‘antipodal effect’ affecting the syntheses of para-carborane derivatives. New two-step preparations of 1-ethynyl- and 1,12-bis(ethynyl)-para-carboranes have been developed using a more readily prepared
C-12-Cu-1,12-C2B10H10 and the bromoalkyne. The ethynyl group at the cage carbon C(1) strongly influences the chemical reactivity of the cage carbon at C(12)—the first example of the ‘antipodal effect’ affecting the syntheses of para-carborane derivatives. New two-step preparations of 1-ethynyl- and 1,12-bis(ethynyl)-para-carboranes have been developed using a more readily prepared ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCMe2OH. The molecular structures of the two C-monoethynyl-carboranes, 1-RC
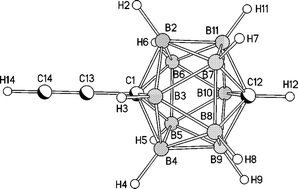
CCMe2OH. The molecular structures of the two C-monoethynyl-carboranes, 1-RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-1,12-C2B10H11 (R = H and Me3Si), were experimentally determined using gas-phase
C-1,12-C2B10H11 (R = H and Me3Si), were experimentally determined using gas-phase ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond distance of 1.233(5) Å. For R = Me3Si (RG = 0.048) a model with Cs symmetry refined to give a C
C bond distance of 1.233(5) Å. For R = Me3Si (RG = 0.048) a model with Cs symmetry refined to give a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond distance of 1.227(5) Å. Molecular structures of 1,12-Br2-1,12-C2B10H10, 1-HC
C bond distance of 1.227(5) Å. Molecular structures of 1,12-Br2-1,12-C2B10H10, 1-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-12-Br-1,12-C2B10H10 and 1,12-(Me3SiC
C-12-Br-1,12-C2B10H10 and 1,12-(Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2-1,12-C2B10H10 were determined by
C)2-1,12-C2B10H10 were determined by ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-1,2-C2B10H11 and the ethene, trans-Me3SiBrC
C-1,2-C2B10H11 and the ethene, trans-Me3SiBrC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CSiMe3Br are also reported.
CSiMe3Br are also reported.

 Please wait while we load your content...
Please wait while we load your content...