Complementarity and countercomplementarity in polynuclear copper(ii) complexes with R2NCH2CH(OH)CH2NR2 (R = H, CH3): crystal structures and magnetic study
Abstract
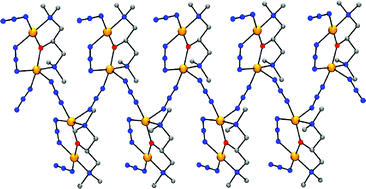
The syntheses, structural characterization and magnetic behavior of five new copper(II) polynuclear compounds with formulae [Cu4(μ2-CH3COO)2(μ-bdmap)2(μ1,5-dca)2(dca)2(H2O)2] 1, [Cu2(μ2-CH3COO)(μ-bdap)(μ1,1,5-dca)(μ1,3-dca)]n2, [Cu4(μ2-CH3COO)2(μ-bdmap)2(μ1,1-NCS)2(NCS)2] 3, [Cu2(μ2-CH3COO)(μ-bdap)(NCS)2] 4 and [Cu2(μ1,3-N3)(μ-bdmap)(N3)2]n5 in which bdmapH is ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with unit cell parameters a = 8.184(5), b = 8.792(2), c = 10.887(2) Å, α = 75.65(2), β = 76.55(3), γ = 74.36(3)°, Z = 2. Tetranuclear complex 3 crystallizes in the triclinic system, space group P
, with unit cell parameters a = 8.184(5), b = 8.792(2), c = 10.887(2) Å, α = 75.65(2), β = 76.55(3), γ = 74.36(3)°, Z = 2. Tetranuclear complex 3 crystallizes in the triclinic system, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with unit cell parameters a = 8.455(4), b = 9.114(9), c = 12.744(8) Å, α = 104.62(8), β = 99.86(6), γ = 106.10(8)°, Z = 1. Dinuclear complex 4 crystallizes in the triclinic system, space group P
, with unit cell parameters a = 8.455(4), b = 9.114(9), c = 12.744(8) Å, α = 104.62(8), β = 99.86(6), γ = 106.10(8)°, Z = 1. Dinuclear complex 4 crystallizes in the triclinic system, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , with unit cell parameters a = 8.15(1), b = 8.18(2), c = 11.44(1) Å, α = 69.39(2), β = 80.36(2), γ = 80.37(2)°, Z = 2. One-dimensional complex 5 crystallizes in the orthorhombic system, space group P212121, with unit cell parameters a = 20.45(4), b = 11.36(3), c = 6.43(1) Å, Z = 4. The magnetic behavior of all the complexes has been checked giving a bulk antiferromagnetic coupling in all the cases with |J| values in the range 109–144 cm−1 for 1–4. Compound 5 is diamagnetic in the 2–300 K range of temperatures. The found J values for 1–5 can be justified from the structural data taking into account the orbital countercomplementarity for 1–4 and the orbital complementarity for 5.
, with unit cell parameters a = 8.15(1), b = 8.18(2), c = 11.44(1) Å, α = 69.39(2), β = 80.36(2), γ = 80.37(2)°, Z = 2. One-dimensional complex 5 crystallizes in the orthorhombic system, space group P212121, with unit cell parameters a = 20.45(4), b = 11.36(3), c = 6.43(1) Å, Z = 4. The magnetic behavior of all the complexes has been checked giving a bulk antiferromagnetic coupling in all the cases with |J| values in the range 109–144 cm−1 for 1–4. Compound 5 is diamagnetic in the 2–300 K range of temperatures. The found J values for 1–5 can be justified from the structural data taking into account the orbital countercomplementarity for 1–4 and the orbital complementarity for 5.

 Please wait while we load your content...
Please wait while we load your content...