A chelating β-diketonate/phenoxide ligand and its coordination behavior toward titanium and scandium
Abstract
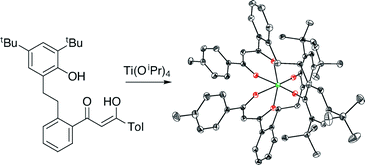
Dibenzoylmethane derivatives with one (L1H2) or both (L2H3, L3H3) benzenes linked at their ortho positions to 4,6-di-tert-butylphenol moieties by two-carbon linkers have been synthesized. The mono-β-diketone-monophenol ligand L1H2 is metalated by titanium alkoxides to form the homoleptic complex (L1)2Ti and heteroleptic complexes (L1)Ti([OCH2CH2]2NR) (R = H, CH3), and reacts with Cp3Sc to form CpSc(L1). These are the first examples of complexes of a β-diketonate ligand which is further chelating to a single metal center. Crystallographic analysis of (L1)2Ti indicates that the 10-membered ring allows chelation of the phenoxide with little strain, and both fac and mer geometries are accessible in solution. Protonolysis of the second cyclopentadienyl ring of Cp3Sc appears to take place by an indirect, Cp3Sc-catalyzed pathway.

 Please wait while we load your content...
Please wait while we load your content...