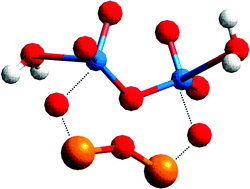
The effect of the point of zero charge (PZC) of the support oxide (Al2O3, Nb2O5, SiO2 and ZrO2) on the molecular structure of hydrated vanadium oxide species has been investigated with EXAFS spectroscopy for low-loaded vanadium oxide catalysts. It was found that the degree of clustering (i.e., the V⋯V coordination number) and the V⋯V distance increase with decreasing PZC of the support oxide; i.e., Al2O3 (8.7) < ZrO2 (7) < Nb2O5 (3.3) < SiO2 (2). Upon hydration the silica-supported vanadium oxide exhibited a clear alteration in the position of the oxygen atoms surrounding the central vanadium atom and the number of oxygen atoms around vanadium increased to five. In contrast, only minor changes in the molecular structure were detected for the alumina-, niobia- and zirconia-supported vanadium oxide catalysts. Based on a detailed analysis of the EXAFS data a semi-quantitative distribution of vanadium oxide species present on the surface of the different support oxides can be obtained, which is in good agreement with earlier characterization studies primarily making use of Raman spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...